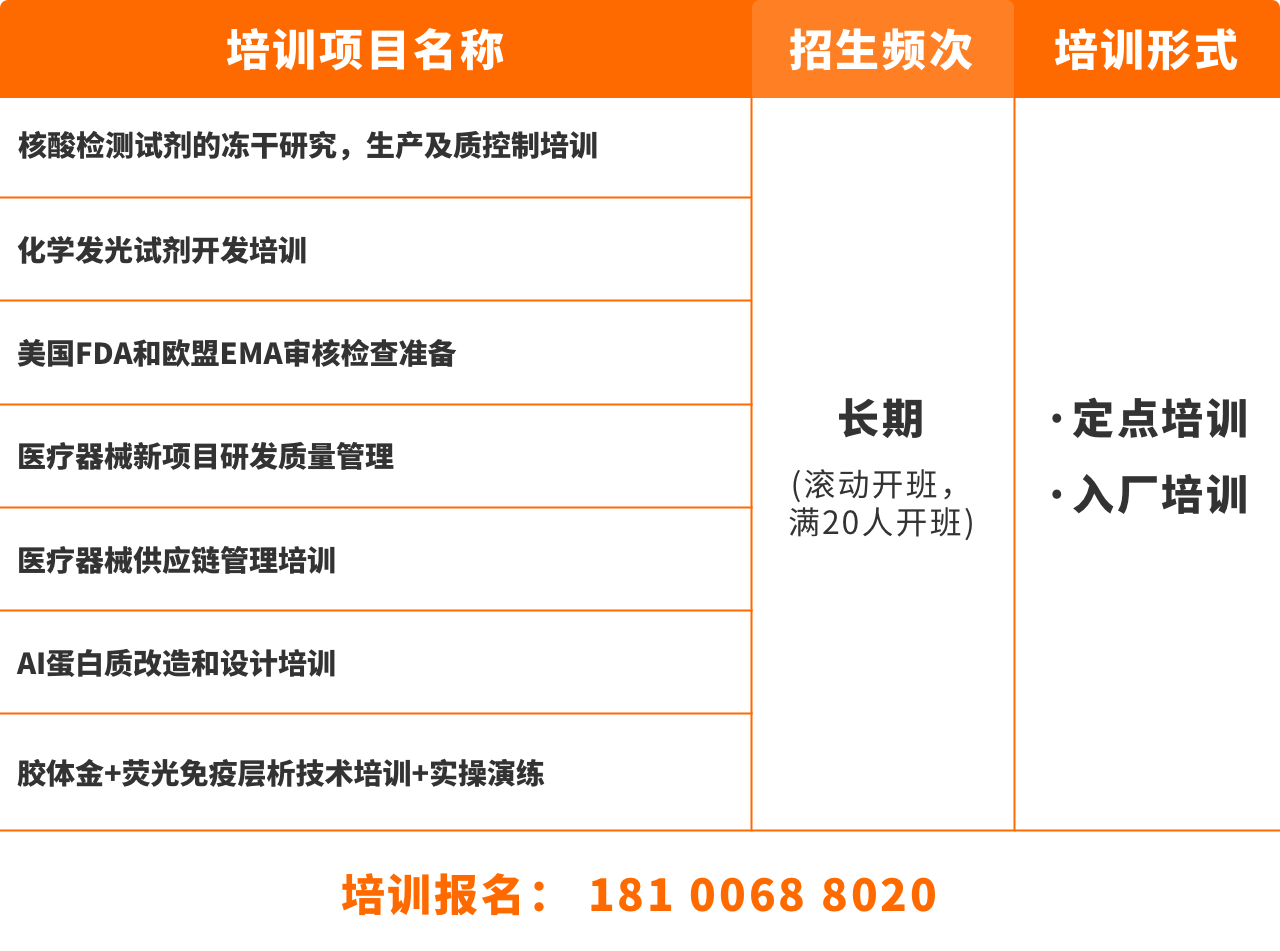
美国FDA和欧盟EMA审核检查准备-专题培训
- 会议地址
- 江苏省苏州市工业园区定点培训班/入厂培训
- 会议时间
- 2024-08-24~2024-08-25
- 会议类型
- 培训会
- 所属行业
- 实验仪器>其它
- 关键词
培训简介
培训主题:美国FDA和欧盟EMA审核与检查,企业准备事项分析与建议
培训时间:2024.08.24-25日
培训地点:苏州培训形式:定点培训班/入厂培训(英文培训/专业翻译支持)
主办单位:桔园平台、Global PharmaDevice Solutions、常州中美歆新
培训面向群体
制药、生物制品、医疗器械、IVD行业:总经理、CEO、运营总监、制造/生产经理、QA经理/主管、QC经理等。
培训讲师介绍
Mr. Matthew Azuh is the Founder and CEO of Global PharmaDevice Solutions (GPDS) located in Chicago Area, IL USA. GPDS is a global regulatory consulting firm and product sterility testing laboratory serving the pharmaceutical, biologics, and medical device industry across the globe. Mr. Azuh has been in the industry for over 34 years with thorough and in-depth knowledge of regulatory compliance requirements of the FDA (USA), TGA (Australia), EMA/PMDR (EU), CMDR (Canada), PMDA (Japan), ANVISA (Brazil), SSA (Mexico), ISO 13485, and ISO 9001. Areas of leadership and expertise include ANDA, NDA, 510K, CE Marking, CLIA, PMA petition/submission, Consent Decree Management, Warning Letter Remediation, Quality System Architecture and Implementation, GxP Auditing, Domestic/international cold chain design and validation, CAPA Remediation, GMP/GCP training, Time and Extent Application (TEA) for foreign/domestic marketed drugs, Process, Utility, and Software Validation, Aseptic Processing, and Project Management.
培训课程收益
1.了解如何为举办FDA、EMA和其他机构的检查做好充分准备;
2. 知道如何保持相对良好的GxP合规性状态;
3. 执行和主持模拟检查;
4. 成功的检查结果;
5. 能够及时推出产品;
深度咨询服务
- GxP审核培训(GxP Audit Training);
- FDA/EMA模拟检查(FDA/EMA Mock Inspections);
- QMS补救(QMS Remediation);
- 药物,医疗器械,IVD监管注册和提交(Drug, Medical Device, IVD regulatory registration and submission);
- 质量体系的开发和实施(Quality System Development and Implementation);
已合作客户
1.辉瑞制药(Pfizer Pharmaceutical);
2.罗氏诊断(Roche Diagnostics);
3.博士伦(Bausch & Lomb);
4. Quest 诊断(Quest Diagnostics);
5. 默克制药(Merck Pharmaceuticals);
6. 梅里埃生物公司(BioMerieux Inc);
7. Apellis制药公司(Apellis Pharmaceuticals);
8. 礼来制药(Eli Lilly Pharmaceuticals);
9. 安进制药(Amgen Pharmaceuticals);
10.库里南肿瘤学(Cullinan Oncology);
11.菲利普斯公司(Phillips Corp);
12.雅培实验室(Abbott Labs);
13.直觉公司(Intuitive Fluorescence Imaging);
14.美国国立卫生研究院(US National Institute of Health);
15.C4疗法(C4 Therapeutics);
16.卡普里科治疗药物(Capricor Therapeutics);
17.登士柏西诺德(DentsPly Corp);
18.Defender 制药(Defender Pharmaceuticals);
- 55
- 点赞
- 复制链接
- 举报